In India, the survival rate for breast cancer remains lower than in many high-income countries. Contributing factors include late diagnosis, limited screening uptake, and a younger average age at onset. Standard breast cancer treatment may involve one or more modalities, including surgery, chemotherapy, hormone (endocrine) therapy, radiation therapy, immunotherapy, and targeted therapy. Outcomes have improved significantly over the past two decades. Yet, researchers worldwide continue to explore new, more precise and less toxic therapeutic approaches.
Historically, the primary surgical treatment was radical mastectomy, which involved the removal of the entire breast along with axillary lymph nodes and sometimes chest muscles—often resulting in significant physical and psychological impact. Advances in diagnostics and surgical technique — including digital breast tomosynthesis (3D mammography) and breast-conserving surgery (lumpectomy) paired with radiation therapy — now allow many patients to preserve breast tissue while achieving excellent tumour control.
Radiation therapy techniques have evolved considerably. Options such as partial breast irradiation, brachytherapy, intraoperative radiotherapy, and intensity-modulated radiotherapy (IMRT) now help limit radiation exposure to healthy tissue and improve cosmetic outcomes.
Chemotherapy protocols — using single or combination drugs tailored to cancer subtype and stage — remain an important component of treatment. Anthracyclines, for example, act by intercalating with DNA, inhibiting topoisomerase II, and preventing cancer cell replication, though they may cause notable systemic toxicity, and drug resistance can occur. Chemotherapy may be administered before surgery (neoadjuvant) to shrink tumours or after surgery (adjuvant) to prevent recurrence.
Hormonal therapy is widely used for hormone receptor-positive cancers, the most common breast cancer subtype. It works by blocking the pathways for estrogen or progesterone that are necessary for tumour growth. Medications such as tamoxifen or aromatase inhibitors are standard treatments. In select cases, surgical ovarian suppression may be used.
Immunotherapy is increasingly relevant in breast cancer treatment. Immune checkpoint inhibitors — particularly for triple-negative breast cancer — have been approved and are providing meaningful benefit for some patient groups. CAR-T cell therapy remains experimental in breast cancer and is currently being studied in early-stage clinical trials.
Targeted therapies, including antibody-drug conjugates (ADCs), represent one of the most promising advances in modern medicine. ADCs link monoclonal antibodies that recognise specific cancer cell proteins (such as HER2) to potent cytotoxic drugs, enabling highly targeted destruction of tumour cells. Examples such as trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd) are already in clinical use for HER2-positive breast cancer.
Newer therapies
Significant progress in molecular biology and cellular oncology has led to a new class of emerging cancer therapies focused on precision and reduced toxicity.
Photothermal therapy (PTT) is one such experimental approach. It involves introducing photothermal agents — often nanoparticles—into the tumour. When exposed to near-infrared (NIR) laser light, these agents generate heat, selectively destroying cancer cells through thermal ablation. Both organic nanoparticles (e.g., cyanine dyes and porphyrins) and inorganic materials (e.g., gold and carbon-based nanostructures) are being studied. Current challenges include limited tissue penetration of NIR light and potential damage to surrounding healthy tissues — research into fibre-optic-guided delivery and improved targeting aims to overcome these limitations.
Personalised treatment strategies are gaining prominence. Antibody-drug conjugates exemplify this direction, enabling the selective killing of cancer cells while sparing healthy tissue. Indian research centres, including Tata Memorial Hospital, are actively investigating novel nanoscale delivery systems, including marine-derived biopolymers, to enhance drug targeting and reduce toxicity — currently in preclinical evaluation.
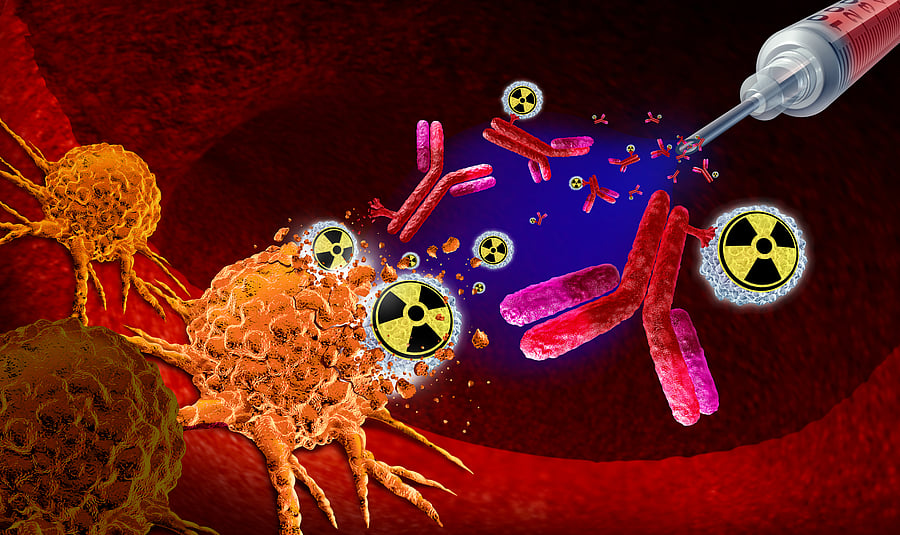
Another area of promising research involves radiopharmaceuticals. The protein Nectin-4 is overexpressed in certain breast cancers and is a potential biomarker for disease progression. Targeted molecules engineered to bind securely to Nectin-4 are being developed, and some research teams have paired these molecules with alpha-emitting radionuclides such as Actinium-225 to deliver highly localised radiation directly to tumour cells.
Most current radiopharmaceutical treatments use beta-emitting isotopes such as I-131, Y-90, Lu-177, and Sm-153. Alpha-emitters, however, offer higher linear energy transfer and very short penetration ranges — allowing more effective destruction of tumour cells while sparing adjacent tissue. Actinium-225, with a half-life of about 10 days, is considered well-suited for this approach. Production of Actinium-225 remains limited worldwide, though it can be obtained through separation (“milking”) from thorium-229 or by proton-induced spallation of thorium-232. India’s long-standing thorium-fuel research and the operation of the U-233-fuelled KAMINI reactor underscore the nation’s scientific strength in this domain.
Advances across biology, chemistry, materials science, and nuclear medicine are accelerating the development of cancer therapies that are more effective, precise, and personalised. Many future treatment strategies are likely to be guided by an individual patient’s unique genetic, environmental, lifestyle, and tumour factors.
Artificial intelligence will play a major role — from imaging and risk prediction to drug discovery, treatment planning, and post-treatment monitoring. As clinical research continues to advance, the gap between discovery and practice is expected to shorten.
(The author is a former director of radiological safety at the Atomic Energy Regulatory Board)