Paediatrician Sivaranjani Santosh
Credit: X/@dr_sivaranjani
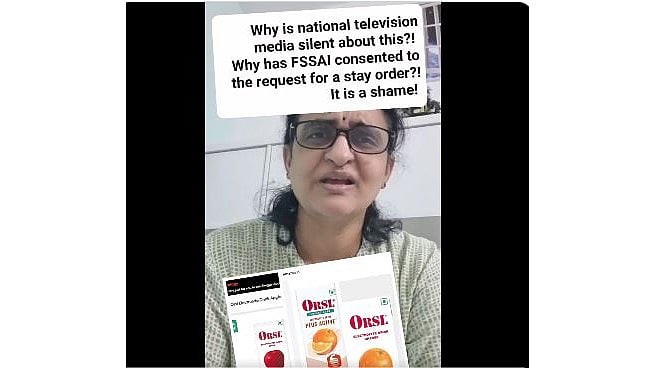
A paediatrician from Hyderabad, Sivaranjini Santosh, has criticised the Food Safety and Standards of India (FSSAI) for allowing the disposal of high-sugar ORSL (Oral Rehydration Solution) drink stocks, calling it a "shameful act".
In a video posted by her, the doctor has urged the authorities to ensure only "WHO-recommended ORS formulations" are permitted in pharmacies, hospitals, schools, and online platforms.
She also called for strict implementation of the October 15 FSSAI order, which prohibits the use of 'ORS' on the labels of any beverage that does not meet medical standards.
“ORS should never be allowed on labels except for WHO-recommended formula ORS, even if sold in supermarkets or quick-commerce platforms,” she wrote in her post.
However, the FSSAI denied the allegation, citing that permission was not given by the authority for the sale or disposal of ORS stocks. The regulator called it a “misrepresentation of facts”, stating that claims suggesting FSSAI’s approval are false, and asked people to refer to the official court order.
The paediatrician's post on X got support from many social media users, who said such misleading marketing practices, especially in the healthcare sector, should be stopped.
A social media user, Dr Alok Gupta, said, "We are 1 voice for allowing WHO ORS in the market with immediate effect. The Rouges cannot be allowed to harm the Indian Children any longer after the law has accepted the mischief done by surrogate marketing."
Another user wrote, "This is basically (ORSL + FSSAI) vs People of India case. This case will be testament to the system we have in place that denies to protect people from harms way."
