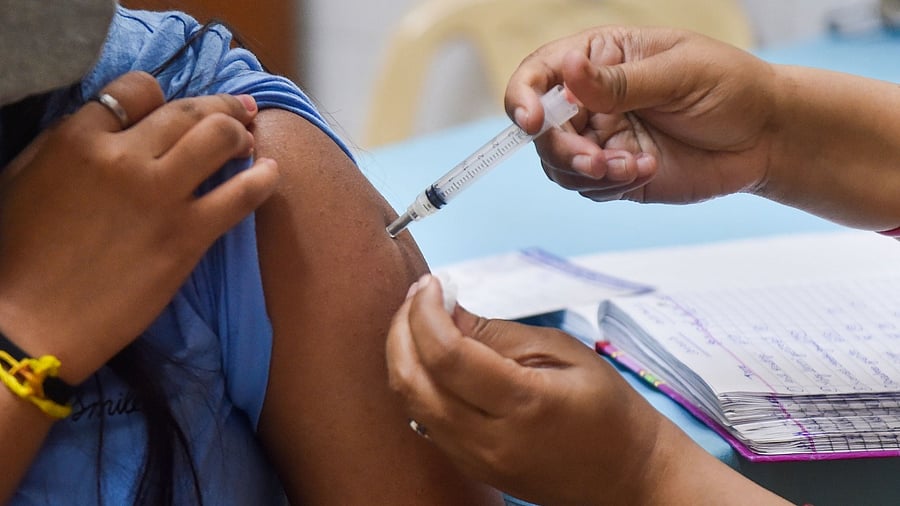
The Standing Technical Sub-Committee of the NTAGI has recommended inclusion of the Serum Institute's Covovax in the national Covid-19 vaccination programme for children aged 12 to 17 years, sources said on Friday.
India's drug regulator had approved Covovax for restricted use in emergency situations in adults on December 28 last year and in the 12-17 age group, subject to certain conditions, on March 9.
"The Covid-19 working group of the NTAGI (National Technical Advisory Group on Immunisation) had earlier reviewed data related to Covovax and okayed it. The NTAGI's Standing Technical Sub-Committee which met on Friday has recommended that the vaccine can be used for 12-17 years age group," an official source said.
Serum Institute of India (SII) Director for Government and Regulatory Affairs Prakash Kumar Singh had written to the Union Health Ministry recently, requesting for Covovax's inclusion in the immunisation drive for those 12 years and above.
Singh had stated that the Pune-based firm wanted to provide Covovax to private hospitals at Rs 900 per dose plus GST and was waiting for directions to supply it to the Centre. However, the price of the vaccine for the government was not mentioned.
The country began inoculating children aged 12-14 from March 16.
Watch latest videos by DH here: