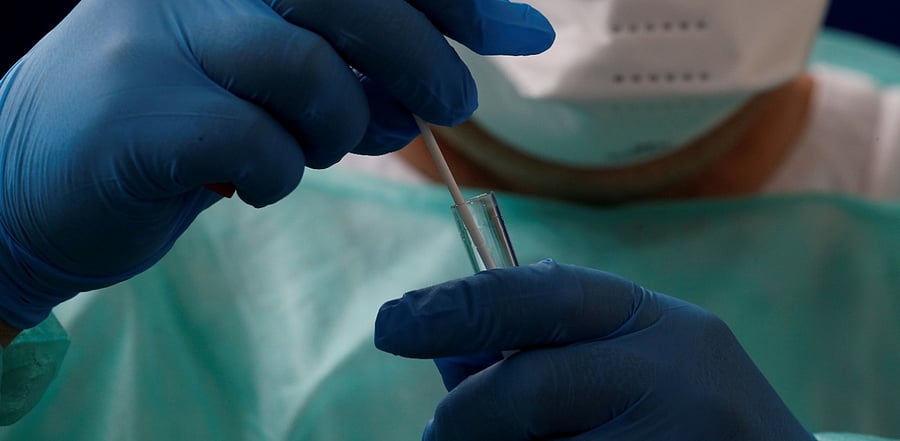
A recent clinical trial provides evidence that 3D-printed alternative nasal swabs work as well, and safely, for Covid-19 diagnostic testing as the commercial synthetic flocked nasal swabs.
The researchers developed 3D-printed nasopharyngeal (3DP) swab as a replacement of the flocked nasopharyngeal (FLNP) swab and concluded that 3D printing technology is a viable and cost-efficient alternative to address the FLNP swab supply shortages.
The authors of the trial report note that throughout the course, although slight nasal bleeding after completion of both swabs was reported, there were no other adverse reactions. The results were published on September 10 in Clinical Infectious Diseases.
The trial was led by the University of South Florida Health (USF Health) Morsani College of Medicine and its primary hospital affiliate Tampa General Hospital (TGH).
The material cost per 3DP swab ranges from $0.25-$0.46, while the commercial FLNP swabs cost approximately $1.00 per unit.
In a commentary on 3D printing in medicine, Dr Frank Rybicki wrote that among all parts 3D printed during Covid-19, nasopharyngeal swabs have received the most attention, with participants ranging from humanitarians to charlatans.
"The authors should be congratulated for staying on the right side of the curve, and for their perseverance, leadership, scientific rigour, and goodwill," he said.
Earlier in March, New York-based hospital system Northwell Health said it has started to make its own nasal swabs using 3D printing, enabling it to produce thousands of swabs a day that can be used in testing for the coronavirus.
(With agency inputs)
